Diagnostics
Novel Diagnostic Assay: Lateral Flow Assay under development for Babesiosis
Multi-Antigen immunoassay is under development for Babesiosis. Our innovations are guided by phage display cDNA technology and serology-based immunoassays. Currently, we are developing a Lateral Flow Test (LFT) for rapid point-of-care detection of babesiosis.
Multi-Antigen Immunoassay for Babesiosis
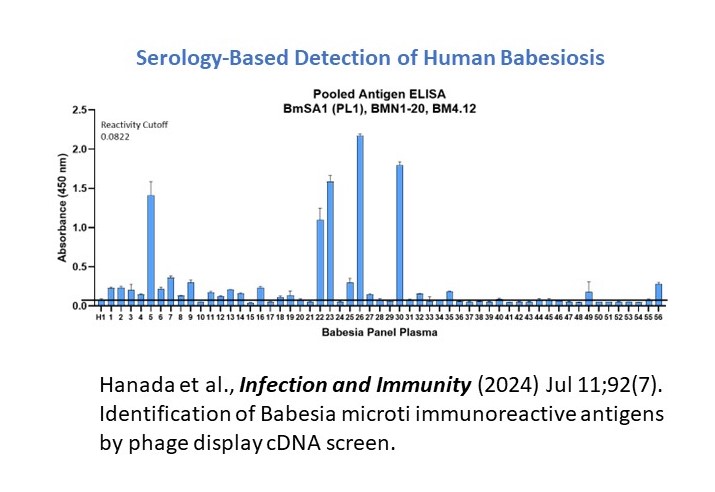
We use phage display technology to generate cDNA libraries designed to identify critical host-pathogen interactions. Babesia microti, a malaria-like parasite, causes Babesiosis, a tick-borne disease. Babesia microti and related species invade red blood cells. Using blood plasma from patients afflicted with Babesiosis, three key parasite antigens were identified from the cDNA library screens. Defined recombinant proteins were used to develop a highly specific diagnostic assay for human Babesiosis. Predictive algorithms and modeling approaches are currently utilized to generate synthetic versions of the stable peptide epitopes. A pool of immunogenic peptides is used for the large-scale production of Multi-Antigen Immunoassays for Babesiosis (MIB™ kits) covering a variety of Babesia infections.